Arsenic – #1 King of Poisons
- World Health Organisations – Top 10 Chemicals of concern include Arsenic (June 2020)
- Department of Health and Human Service and Agency for Toxic Substances and Disease Registry (ATSDR) rank Arsenic number one among substances present in the environment that pose the most significant threat to human health.
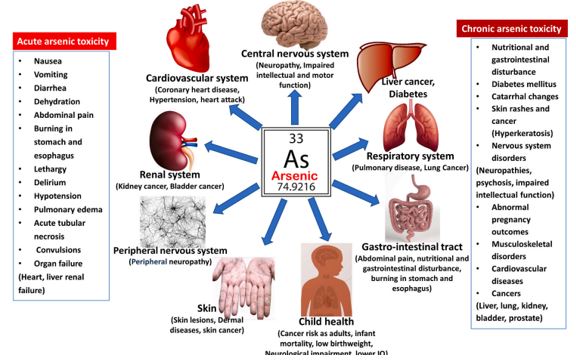
- The International Agency for Research on Cancer (IARC) classifies arsenic and inorganic arsenic compounds as “carcinogenic to humans.” This is based on sufficient evidence in humans that these compounds can cause: Lung cancer, Bladder cancer, skin cancer. IARC also notes links in some studies to: Kidney cancer Liver cancer Prostate cancer. For more detailed information, see the IARC monograph Arsenic and Arsenic Compounds.
Key Take Aways
TheNumber 1 King of Poisons (ATSDR) a significant heavy metal or environmental toxin
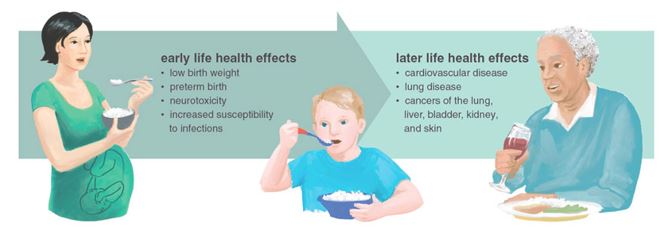
Many studies show that chronic low-level Arsenic exposure is a risk factor for ADHD and Autism disorders. According to the Australian Government – Health Direct website 1 in every 20 children now has ADHD.
An important 2019 Norwegian study provides a comprehensive clinical evaluation methodology for managing the risk of low-level arsenic burdens on neurodevelopment in children. The framework shifts away from a “one-exposure-for-one-health effect” to one that acknowledges the complex factors. It provides the answers clinicians require – detailing the assessment method and appropriate biomarkers.
Arsenic also is a risk factor for cardiovascular disease, diabetes, respiratory diseases, Gut disturbances and diseases, immune issues and more.
Hair Tissue Mineral Analysis HTMA is an excellent method to assess exposure
Natural Detoxification antagonists include: Selenium, Sulphur, Vitamin E & Beta-Carotene
Arsenic Heavy Metal Contamination
There are two general forms of arsenic Organic (as arsenic compound containing carbon) and Inorganic (which research indicates is more toxic and associated with greater health effects).
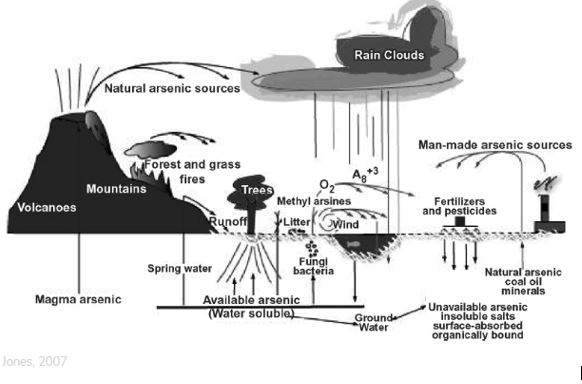
Contaminated drinking water globally is one of the most significant routes of contamination. Arsenic in Australian & New Zealand water may be at high levels as it can be dissolved from natural rock. There are a significant number of areas (not just near mining sites) where the water is contaminated. (Royal George, Tas; Uralla & Stuarts Point, NSW; Urandangie QLD as can be seen on Australian Drinking Water Map. The diagram below shows possible contamination pathways including old volcanoes, forest fires, rock, vapour etc
In Australia, food is the largest source of arsenic with 77.5% of exposure to arsenic in children originating from food and water. The highest levels of arsenic (in all forms) in foods can be found in seafood, rice, rice cereal (and other rice products), mushrooms, and poultry, although many other foods, including some fruit juices, can also contain arsenic. The NSW Food Authority has previously conducted a testing program of metals in fish. In this program, elevated total arsenic levels were found in some fish, mainly angel fish, angel shark, flake, or fish cocktail.
Less information is available on the type and level of arsenic present in seaweed. International food regulatory agencies and Food Standards Australia New Zealand have issued warnings about the inorganic arsenic content of hijiki seaweed.
The Australia New Zealand Food Standards Code includes a maximum level of total arsenic in cereal grains and milled cereal products, including rice, of 1 mg/kg, with no limit specified for inorganic Arsenic (FSANZ 2017). This level is higher than the current Codex Alimentarius Commission (United Nation’s Food and Agriculture Organization) maximum level for inorganic Arsenic of 0.2 mg/kg and 0.35 mg/kg, for polished and husked rice, respectively (CCCF 2017).
The European Commission (EC 2015) set a maximum level of 0.2 mg/kg inorganic Arsenic for polished rice, 0.25 mg/kg for husked and parboiled rice, 0.3 mg/kg for rice crackers and rice cakes and 0.1 mg/kg for rice destined for the production of infant and young children’s food. [2]
The FDA and other agencies testing revealed that rice-based products, including noodles, rice cakes, rice cracks and chips contained arsenic at levels comparable to those of rice itself, which greatly affects, individuals with dietary restrictions—such as those on gluten-free diets, diets where rice-based products are recommended (eg. to reduce cholesterol), or individuals following diets that encourage the consumption of complex carbohydrates like brown rice.
Researchers from the Dartmouth Toxic Metals Superfund Research Program (UK) reported on the high arsenic content of organic brown rice syrup, a high-fructose corn syrup alternative found in many health foods, including cereal bars, milk formula, and energy bars or shots.
A recent Australian study at RMIT (2020) looked at 39 rice products for babies and toddlers found in Australian supermarkets, including milk formula powder, cereal, crackers and pasta made from brown, white, organic and non-organic rice. The research found 75% of the products had levels of inorganic arsenic above the EU standard. [4]
Arsenic contamination in the Australian environment has been derived from a range of diverse industries (manufacturing: fertilizer, paint, electronics; gas works; mining; power stations; transport; and timber). Arsenic has been widely used in the agricultural industry as both a pesticide and herbicide as well which has resulted in widespread and elevated levels of contamination throughout the environment. [5]
The mining industry has also contributed widely to the incidences of arsenic contamination. Historical Gold mining sites, especially in Victoria has left a legacy of contamination relating to the method of extraction.
Arsenic pesticides used in orchards have created significant contamination, along with arsenic-based chemicals used as tickicides in livestock dips to control ticks, fleas and lice in Australia [6]. Many of the arsenic-based pesticides were widely used in Queensland, New South Wales and South Australia, from the early 1900s to 1955 and were locally manufactured. [7,8] Studies looked at 1607 former cattle dip sites of which 1041 were still in operational use in 1990 with heavy soil contamination, (with some of those now being in new residential areas of the ACT).
Arsenic-based herbicides were widely used in Australia to control grass growth along railway corridors. Extensive use of As-based herbicides has resulted in the contamination of many former railway corridors in South Australia. These former railway corridors pass through a major cereal and vegetable growing region in South Australia! Some of the contaminated soils are currently being used for crop production and there is increasing pressure from farmers to release the currently fenced railway corridors for crop production in the mid-north of SA.[5]
Toxic Mechanisms
 [9]
[9]
Primary Arsenic absorption is through the small intestine. Other routes of exposure are from skin contact and inhalation. Following distribution to many tissues and organs in the body including the lungs, heart, kidneys, liver, muscles, and neural tissue, Arsenic is metabolized to monomethylarsonic acid (MMA) and dimethylarsinic acid (DMA). DMA is the predominant form the urinary excretion [10]).
Acute and chronic Arsenic toxicity is related to the dysfunctions of numerous vital enzymes. Like the other heavy metals, Arsenic can inhibit sulfhydryl group containing enzymes which leads to their dysfunction. Moreover, it inhibits the pyruvate dehydrogenase by binding to the lipoic acid moiety of the enzyme. Pyruvate dehydrogenase inactivation can block the Krebs cycle and inhibits oxidative phosphorylation. As a result, ATP production decreases, resulting in cell damage [11].
Furthermore, the damage of capillary endothelium increases vascular permeability, leading to vasodilation and circulatory collapse.
Arsenic & Development Toxicity
An excellent study in 2020 Developmental toxicity of arsenic: a drift from the classical dose–response relationship in Springer [12], looked at creating a model to understand the real-life risk and impact assessments firstly considering:
1 Co-exposure to multiple toxins, then
- Individual genetic then
- Nutritional predisposition,
- The stage of neurodevelopment in the child
The study documents a comprehensive approach to assessing chronic low-level arsenic exposure which should include the appraisal of concurrent exposure to multiple toxins, individual nutritional status, together with available information of genetic predisposition to the increased individual susceptibility. The impact assessment should also extend across the neurodevelopmental continuum as much as possible. Hair and nails were identified as the best method for assessing exposure.

The study further discussed that the co-occurrence of high exposure to arsenic, lead, mercury, cadmium, and aluminium, together with low hair levels of selenium, zinc, molybdenum, sulphur, and phosphate, also in well-nourished children which compromises the toxin biotransformation processes. The support of methylation and antioxidant capacity is vital to mitigate the arsenic toxicity. Further lifestyle changes include adequate hydration, avoiding constipation, regular exercise, ensuring good sleep quality to enhance excretion through urine, skin, and bile routes, and preventing the reabsorption of toxins.
Arsenic & Immunity
Arsenic also has considerable adverse effects on both the innate and the adaptive immune systems as shown in the diagram below.
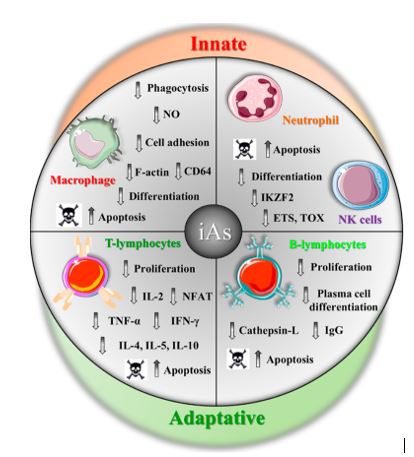
Innate and immune cell images were obtained from Servier Medical Art.
Detoxification
The most effective and well-known treatment for arsenic poisoning is chelation therapy. The most common chelation therapy agents are BAL, DMPS, DMSA, and D-penicillamine. However, these chelation therapy agents are mainly effective in high-level acute arsenic poisoning and have several undesirable side effects. [17]
Many reports have demonstrated that there is a relationship between poor nutritional status and arsenic toxicity, and malnutrition may increase the susceptibility to arsenic toxicity [15, 16 17 18}
Therefore, proper diet and nutrition can influence arsenic methylation, bioavailability and metabolism as well as reduce its toxicity. A high-protein diet rich in vitamins A (beta-carotene), E, and C, as well as supplements in the form of vitamins A, E, C, antioxidants, folic acid, are beneficial and may help to recover from early symptoms [19].
Natural detox: Several studies have revealed that elevated levels of glutathione, proper micronutrients such as zinc, and selenium, as well as some vitamins, tea, and N-acetylcysteine, can reduce arsenic-induced toxicity possibly by reducing the availability or formation of toxic mono-methylated species.
Conclusion
A Hair Tissue Mineral Analysis (HTMA) from InterClinical Laboratories enables practitioners to accurately assess not just toxic metals, (such as arsenic) but all so essential nutritional minerals. Thirty-seven minerals are assessed and quantitative results are provided in parts per million and shown graphically on a (flattened) bell-shaped curve. Ideally, the hair sample is 1 – 4cm long (cut closest to the scalp) and enough to cover a 20-cent coin or fill a teaspoon. The hair sample is dissolved using a proprietary acid digestion solution and then analysed via an ICP-MASS spectrometer in the same way minerals are assessed in the blood. The test is the most cost-effective, non-invasive, reliable method of assessing patient nutrient and heavy metal mineral status.
References
