Copper Deficiency & Excess – Mastering Minerals 10 Masterful Facts

InterClinical eNews March 2020, Issue 102
Characteristics of Copper
Copper is an essential micronutrient. The human body contains approximately 100 mg. It is a co-factor for many redox enzymes with ceruloplasmin being the most abundant Cu-dependent ferroxidase enzyme (with an important role in iron metabolism). Copper is involved in a myriad of biological processes including haemoglobin synthesis, iron oxidation, oxidant defence, neurotransmitter synthesis, cellular respiration, pigment formation, connective tissue and bone formation, and immune function. (1, 2)
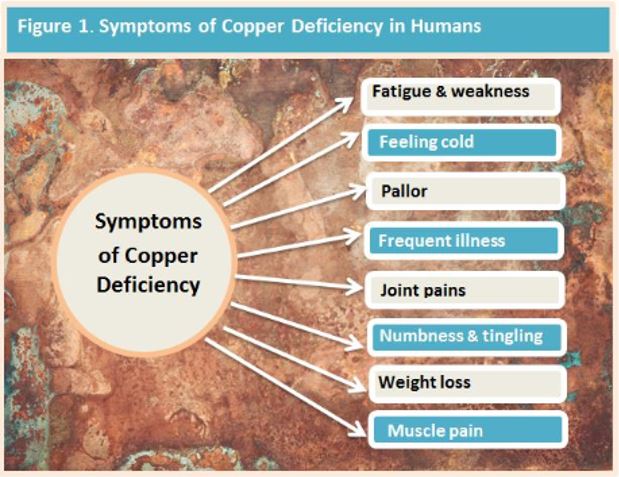
Severe copper deficiency is easily diagnosed due to a wide range of clinical signs resulting from the disturbance of cuproenzymes (cellular transporters). However, identifying mild deficiency is difficult and can result in adverse consequences throughout life. (1) An excess, often called copper toxicity or even hidden copper toxicity may be seen, which also has adverse health consequences,
In Australia, the average adult daily intake is approximately 1.7 mg per day. Absorption may be approximately 10% higher in women than men, and in infants, copper uptake from breast milk is almost total. Concentrations tend to increase with age and are generally higher in females than males. This is exacerbated by pre-menopause as a result of estrogen-dependent ceruloplasmin synthesis, secretion by the liver and oral contraceptive use.
Copper levels are also raised by chronic inflammatory conditions, such as rheumatoid arthritis due to ceruloplasmin being an acute-phase protein regulated by inflammatory hormones. (1)
Factors Affecting Cu Homeostasis
At high concentrations, Cu can stimulate metallothionein synthesis. Under normal conditions, Cu exists at extremely low concentrations as the free ion in the cytosol. Cu-binding ligands, therefore, are key regulators in copper movement and adaptation to toxic effects. The ligands protect against toxicity and can help target the proteins for Cu incorporation. Cu-binding ligands include glutathione (GSH), amino acids, ATP, and the recently identified Cu metallochaperones. These ligands have been shown to transport Cu within the cell from one location to another and make it available to intracellular enzymes. (3)
Scurvy
Scurvy-like symptoms have been observed in copper deficiency. There are strong comparisons in the symptoms of Cu deficiency and scurvy. Vitamin C deficiency may impair Cu metabolism to the extent that transport and regulated uptake of it by cells can be compromised. Cu transport from ceruloplasmin is keenly sensitive to ascorbate. (4)
Liver Function
Copper is unique among cations in that the liver regulates its balance, and a slight defect in this regulation could affect serum concentrations.
Hepatocytes are responsible for copper through several mechanisms: (5)
- Incorporation of it into ceruloplasmin and secretion into plasma (5)
- Temporary storage of it into metallothioneins (5)
- Incorporation of it into copper enzymes (5)
- Excretion of it via the bile through ceruloplasmin (5)
Genetic Disorders
Wilson’s disease is a genetic disorder featuring an accumulation of copper mainly in the liver and brain. It is an autosomal recessive disorder caused by mutation of ATP7B gene in chromosome 13. The release of copper from hepatocytes is highly regulated via Cu-ATPase ATP7B gene. (2) Paradoxically, serum and hair tissue testing for Wilson’s disease reveals a decrease in copper.
Menke disease is a rare fatal genetic disease caused by mutations in the protein ATP7A, resulting in severe copper deficiency. (6) ATP7A is required for cuproenzyme biosynthesis and is important in the copper transport pathway. (7,6) Copper replacement therapy administered parenterally may modify the severity, although a definite cure has not been established. (6)
High Zinc Intake: Zn/Cu Relationship
Zinc is another important micronutrient in the human body, acting as a catalyst or cofactor for many enzymatic reactions. Cu-deficiency anaemia secondary to zinc excess was first reported in 1977 and arises from an indirect interaction between the two metals in the intestine. (8) Zinc is mainly absorbed in the jejunum by metallothionein, similar to copper’s absorption and then transported to the liver. (5)
High zinc intake can cause an up-regulation of metallothionein, which has a greater affinity for copper in enterocytes than zinc. This results in decreased copper entry into plasma, since copper is likely to be bound to metallothionein, resulting in increased zinc absorption. (2)
As zinc supplementation is common, clinicians and pathologists should be alert to the serious hematologic effects of zinc-induced copper deficiency and recognize this condition as an avoidable and readily managed cause of anemia and neutropenia. (8)
Conversely, proper copper absorption and metabolism requires an appropriate zinc balance. It has been found that a decrease in serum testosterone is significantly associated with a high level of copper and elevated Cu/Zn ratio in hair tissue. Hair Tissue Mineral Analysis (HTMA) is one of the most powerful ways of assessing zinc status in humans. (14) Dr David Watts has shown that when appropriately sampled, HTMA can also provide a good index of nutritional copper status and reflect liver copper concentrations. (15, 16)
It is important to understand that the ratio of copper to zinc is clinically more important than the concentration of either of these trace elements. (9)
Ceruloplasmin and Citrate used in supplements
The functions of ceruloplasmin are copper transport, iron metabolism, antioxidant defence, tissue angiogenesis, and coagulation. There are a number of compounds that have a strong inhibitory activity on ceruloplasmin, and one of these is therapeutic doses of citrate. The effect of citrate on the oxidation of ascorbate and other substrates by ceruloplasmin was tested and found to be strongly inhibitory. (10)
Copper Anemia
Copper is an important component of ceruloplasmin and hephestin which are ferroxidase enzymes. Ceruloplasmin is essential for iron transfer from monocyte-macrophage to plasma. A deficiency in copper leads to impaired ferroxidase enzymes, which in turn causes impaired haemoglobin synthesis and results in copper-induced anaemia. (2) In a deficiency, iron builds up in the tissue because the body cannot employ it properly. Often, the excess is deposited in joints, contributing to rheumatoid arthritis. (17)
Potassium Deficiency and Cu
Kha1p, a protein-coding gene, is involved in the acquisition of iron and the utilization of copper. This protein has recently been identified as a new factor in respiratory growth and copper, iron and potassium homeostasis. Research suggests that potassium deficiency in Kha1p cells leads to inefficient copper incorporation into ceruloplasmin. (12)

Copper and diet
The copper content in food varies according to local soil concentrations. However, food groups such as offal and nuts, and to a lesser extent cereals and fruit, can be regarded as good sources of copper, while milk and dairy products contain low amounts. (1)
Copper taken in through the diet might be absorbed partially in the stomach, however, the largest portion of ingested copper passes into the duodenum and ileum, where it enters into the mucosal cells lining the intestine by simple diffusion. As a result of complexing with amino acids, organic acids, or other chelators, a high fraction of copper is soluble in the intestinal tract.
Copper transporting adenosine triphosphatase (ATPase) discharges the copper into the serosal capillaries where the copper bonds to albumin and amino acids for transport to the liver via the portal circulation. From the liver, copper is transported to extrahepatic tissue by albumin, amino acids and to a lesser extent ceruloplasmin. (3)
The amount of copper absorbed from the diet can vary considerably depending on other dietary constituents. However, in general, approximately half the copper consumed in the diet is typically absorbed by the gastrointestinal tract. Approximately two-thirds absorbed is rapidly secreted into the bile, with approximately 80–90% of dietary copper typically excreted in the faeces.
Copper homeostasis is primarily regulated at the GI level through biliary excretion, with the kidney excreting only small amounts of copper.
Bioavailability
The bioavailability, or the fraction of copper absorbed from the GI tract, has been shown to be influenced by the age of the individual, the amount of copper in the GI tract, and various dietary components. For example, copper in meat has been reported to be more bioavailable than in vegetables.
However, the higher copper content of a plant-based diet has been consistently shown to compensate for the slightly reduced bioavailability of copper resulting from the presence of phytates and fibre, suggesting that diets low in or devoid of animal products provide adequate amounts of copper. (1)
Dietary differences have been found in patients with Wilson disease. Observations of two Wilson patients on lactovegetarian diets suggest copper bioavailability is reduced by about 25%. The first of two patients on that diet was asymptomatic for 12 years despite having a typical average daily copper intake and no anti-copper therapy.
Determining Cu status
Copper is carefully regulated like iron and several other dietary essential metals. This is because although they are essential, they are potentially toxic because of their chemical redox potential and their ability to participate in free radical reactions. (7)
Within the cell, copper is escorted to specific compartments by metallochaperones. These chaperones deliver copper to its various target destinations. These copper transporters are the subject of some studies as potential biomarkers to assess copper status, although these have not as yet proved useful. (7)
Despite significant effort over several decades, a sensitive and specific Copper status biomarker has yet to be identified. (11) Because Cu homeostasis is carefully regulated, there is some controversy over serum as an indicator of a deficiency unless it is severe. Serum copper status can be virtually unchanged in cases of mild deficiency.
Copper is also tested using serum ceruloplasmin but this is complicated by the fact that ceruloplasmin is an acute phase reactant, meaning that levels of this copper-carrying protein, and levels of copper, increase in response to inflammation, regardless of copper status. (1) One of the most powerful methods for obtaining copper status is Hair Tissue Mineral Analysis. Hair tissue accumulates all the important trace elements and is easily collected, stored and transported. (17)
Conclusion
Copper is an essential micronutrient involved in many critical physiological functions. Multiple factors have contributed to an increase in deficiency seen clinically over the past decades. This deficiency can result in adverse consequences throughout life such as alterations in bones, the cardiovascular system and ongoing neurological and immunological abnormalities. (2)
Establishing the presence of deficiency or excess can be crucial for good health. Since there are many factors that have a huge influence on copper homeostasis, obtaining a comprehensive overview of nutritional mineral levels is of primary concern. Importantly, the ratio of minerals is clinically more meaningful than the concentration of any trace element.
Hair Tissue Mineral Analysis is a non-invasive and easy way to obtain not only copper status, but also many nutritional mineral ratios including Zinc/Copper and Iron/Copper.
For more information on InterClinical Laboratories Hair Tissue Mineral Analysis (HTMA) testing, visit here.
REFERENCES
1. Bost M, Houdart S, Oberli M, Kalonji E, Huneau J, Margaritis I. Dietary copper and human health: Current evidence and unresolved issues. Journal of Trace Elements in Medicine and Biology. 2016;35:107-115.
2. Myint Z, Oo T, Thein K, Tun A, Saeed H. Copper deficiency anemia: review article. Annals of Hematology. 2018;97(9):1527-1534.
3. Committee C, Staff N, Staff C. Copper in Drinking Water. Washington: National Academies Press; 2000.
4. Harris E, Percival S. A role for ascorbic acid in copper transport. The American Journal of Clinical Nutrition. 1991;54(6):1193S-1197S.
5. Squitti R, Ventriglia M, Barbati G, Cassetta E, Ferreri F, Dal Forno G et al. ‘Free’ copper in serum of Alzheimer’s disease patients correlates with markers of liver function. Journal of Neural Transmission. 2007;114(12):1589-1594.
6. Cao B, Yang X, Chen Y, Huang Q, Wu Y, Gu Q et al. Identification of novel ATP7A mutations and prenatal diagnosis in Chinese patients with Menkes disease. Metabolic Brain Disease. 2017;32(4):1123-1131.
7. Prohaska J. Role of copper transporters in copper homeostasis. The American Journal of Clinical Nutrition. 2008;88(3):826S-829S.
8. Irving JA, Mattman A, Lockitch G, Wadsworth LD. Element of caution: a case of reversible cytopenias associated with excessive zinc supplementation. Canadian Medical Association Journal 2003;169(2):129-31
9. Osredkar J. Copper and Zinc, Biological Role and Significance of Copper/Zinc Imbalance. Journal of Clinical Toxicology. 2011;s3(01).
10. Osaki S. Citric acid as the principal serum inhibitor of ceruloplasmin. J Biol Chem 1964;239:364-6
11. Harvey L, McArdle H. Biomarkers of copper status: a brief update. British Journal of Nutrition. 2008;99(S3):S10-S13.
12. Wu X, Kim H, Seravalli J, Barycki J, Hart P, Gohara D et al. Potassium and the K+/H+ Exchanger Kha1p Promote Binding of Copper to ApoFet3p Multi-copper Ferroxidase. Journal of Biological Chemistry. 2016;291(18):9796-9806.
13. Lee S, Kim M, Kim Y, Chun H, Won B, Lee J et al. Low hair copper concentration is related to a high risk of nonalcoholic fatty liver disease in adults. Journal of Trace Elements in Medicine and Biology. 2018;50:28-33.
14. Ikeda T, Higashi A, Matsukura M. Hair Copper and Zinc Concentrations in Handicapped Children Treated with Anticonvulsants. Developmental Pharmacology and Therapeutics. 1983;6(6):381-387.
15. Watts, D. The Nutritional Relationships of Copper. Journal of Orthomolecular Medicine. 1989;4,2.
16. Jacob R, Klevay L, Logan G. Hair as a biopsy material V. Hair metal as an index of hepatic metal in rats: copper and zinc. The American Journal of Clinical Nutrition. 1978;31(3):477-480.
17. Watts DL. Trace Elements and Other Essential Nutrients. 5th Edn. Writers BLOCK, USA.; 1995.
